Overview
IV-Cell universal culture media is a Research-Use Only (RUO), ready-to-use, fully supplemented medium developed specifically to support bone marrow and peripheral blood cell culture for in vitro cytogenetic analysis of hematological disease. IV-Cell is a proprietary culture media that enables simultaneous culturing of all four (4) hematopoietic cell lineages to solve the problem of selective culturing.
For more information please contact us at
[email protected]
For technical support contact us at
[email protected]
or call 1.203.787.7888 ext. 509

Key Features

Increases Clinical Accuracy
More accurate diagnosis by allowing simultaneous culturing of multiple cell lineages.
All-in-one media
Includes: serum protein, media base, growth hormones, enhancers, B-Cell mitogen, T-Cell mitogens, and Plasma-Cell mitogens.

Reduces errors
Reduces errors caused by eliminating media cocktailing of multiple components and errors in selection of growth factors to use per culture.

Improves inventory management
Reduces 5-6 SKUs to one.

Reduces labor costs
Higher banding resolution results in a reduction of approximately 30 mins for each karyotype & increased auto-scanner productivity (see below).

Cost Savings
Facilitates competitive price resulting in higher margins to laboratory.
Request More Information
Benefits of Increased Banding Resolution
Workflow Benefits:
- 50% reduction in quantity of slides “dropped” in order to achieve viable metaphases for analysis
- 50% reduction in analysis time for abnormal cases, resulting in significant cost reduction in tech time
- Increased capacity on auto-scanners due to the reduction of slides scanned per culture – Average auto-scanner has a capacity of 100 slides or 25 cultures (4 slides per culture), with IV-Cell the capacity is increased to 50-100 cultures (1-2 slides per culture)
Estimated Analysis Time*

*: Based on Precipio testing
Clinical Relevance
- The diagnostic process of hematopoietic diseases involves conducting cell culturing by the cytogenetics laboratory to imitate in-vivo conditions. The four groups of cell lineages cultured are:
- Myeloid cells – indicating myeloid neoplasms (MDS, AML, CML)
- B-cells – indicating B-cell neoplasms (B-cell lymphoma, mantle cell lymphoma)
- T-cells – indicating T-cell neoplasms (T-cell lymphoma)
- Plasma cells – indicating plasma cell neoplasms (multiple myeloma)
- When using the non-Precipio products, the cytogeneticist must decide up front which cell lineage to select to be cultured. In most cases, the cytogeneticist can select only one of the cell lines to culture, due to specimen limitation, low cellularity, or cell viability. More often than not, the initial clinical suspicion is not in line with the final diagnosis determined by the pathologist based on the rest of the work up.
- The results under the existing products can be devastating. If the wrong cell lineage is selected, the diagnosis may be compromised (or return a false negative cytogenetics result) because the lab will be culturing and investigating the wrong cells (essentially “going down the wrong path”).
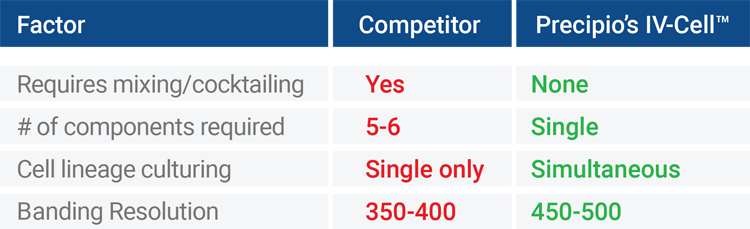
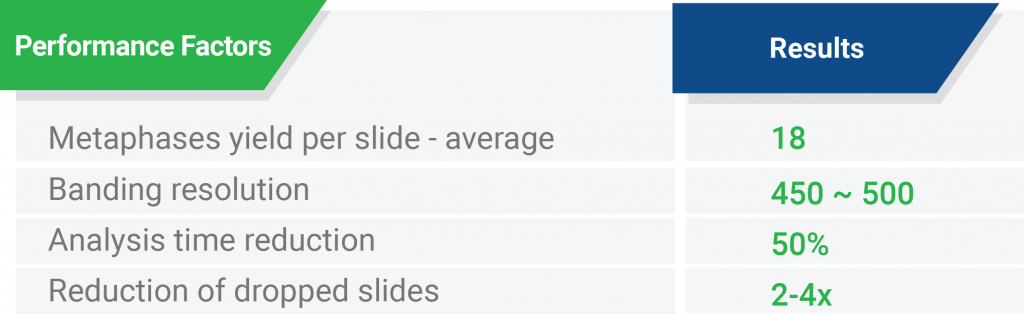
Performance factors based on Precipio internal study
