Myeloproliferative Neoplasms (MPN) Panel Info
The HemeScreen® MPN Panel is a research-use-only assay that detects somatic mutations in targets of interest related to myeloproliferative neoplasms. This reagent set is specifically designed to identify mutations using High-Resolution Melt Analysis in JAK2, MPL, and CALR genes. This reagent set is designed for use by suitably trained personnel testing DNA extracted from whole blood or bone marrow samples using an RT-PCR-based method, or High-Resolution Melt (HRM) Analysis.
Technical Overview IFU SDS ShopDevelopment Of The HemeScreen MPN Panel
Precipio’s own clinical hematopathology laboratory developed the HemeScreen MPN panel to address the issues of turn-around time and accurate, comprehensive molecular testing for gene mutations associated with myeloproliferative neoplasms (MPN) such as polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). By taking a panel approach to gene mutation detection for suspected myeloproliferative neoplasms, Precipio’s clinical hematopathology lab is able to identify incidence of single mutations, mutation changes, and co-expression in a single assay run on one platform. This provides our lab with a more efficient testing workflow and enables rapid turnaround time.
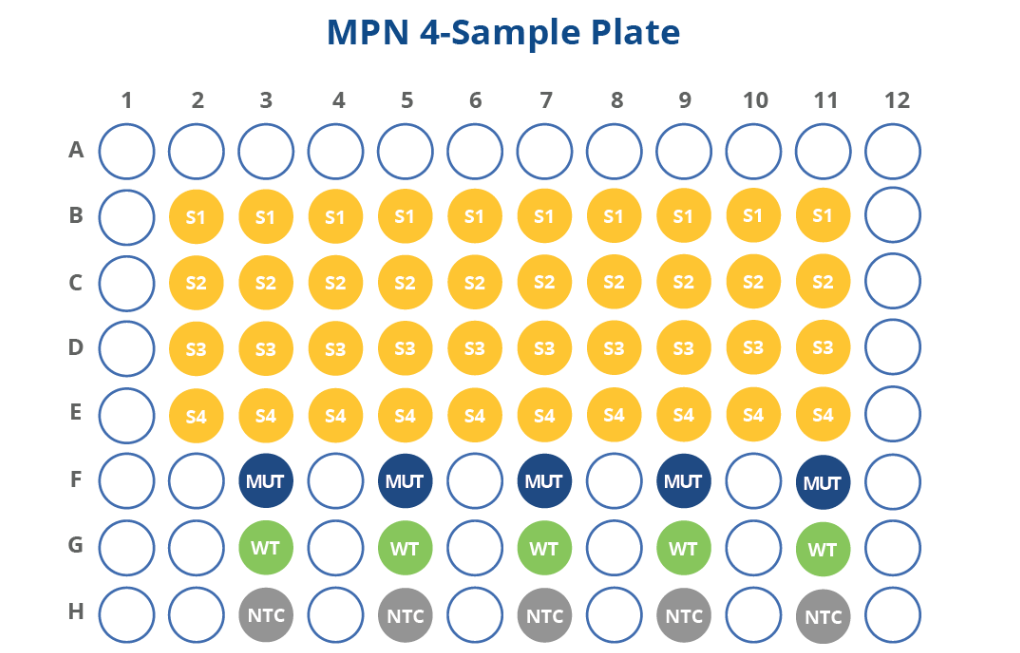
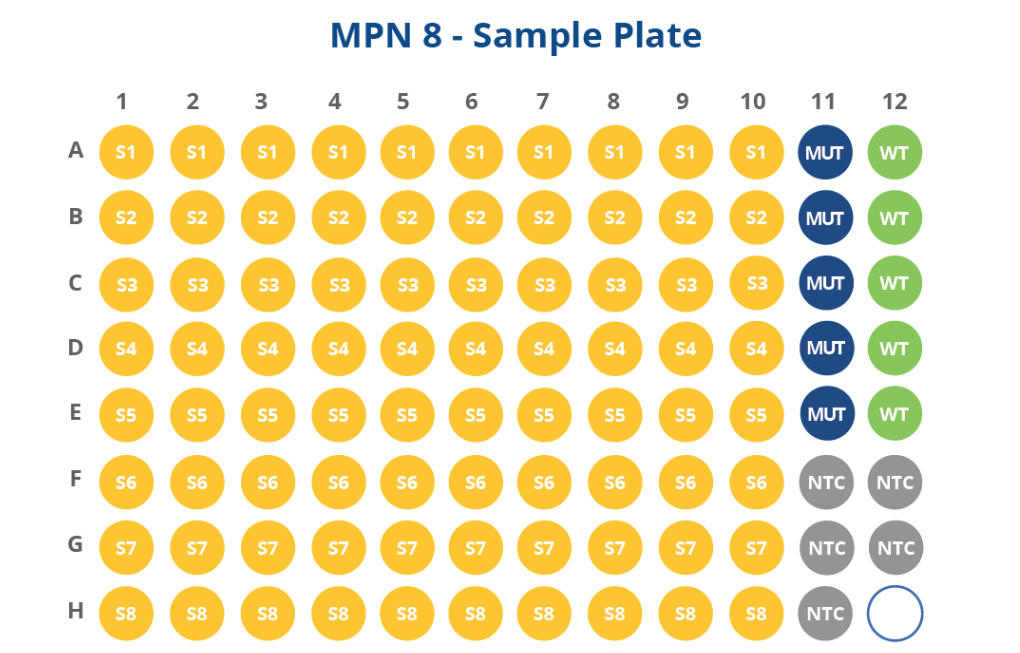
HemeScreen Myeloproliferative Neoplasms (MPN) Assay Configurations
The HemeScreen MPN panel comes pre-plated with optimized primers and embedded mutant, NTC, and wild type controls for driver mutation genes of interest for myeloproliferative neoplasms. This simplifies lab inventory management by providing a complete molecular assay without tracking multiple SKUs of single gene testing assays that require separate control kits.
4-sample pre-plated, 8-sample pre-plated, and vialed free flow configurations are available for the MPN panel. Custom configurations are also possible.
HemeScreen Workflow
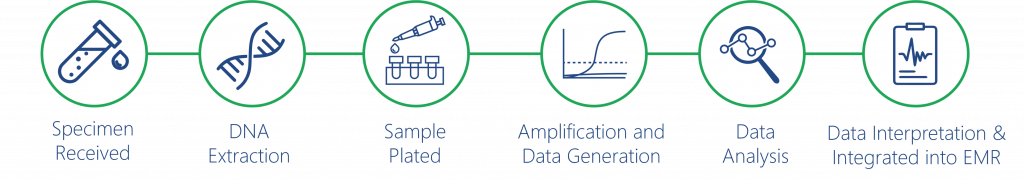
Sample preparation is based off of standard DNA extraction techniques. Most HRM-enabled RT-PCR instruments can run the HemeScreen MPN assay after DNA samples are plated. Data is captured in real time and melt curves can be analyzed to determine the mutation status of JAK2, CALR, and MPL genes.
The shelf life for the HemeScreen assay is 1 year from receipt in the lab.
Data is saved to a secured cloud storage system and also saved locally on the instrument for backup storage.
The total process time to run the assay from DNA extraction to data generation is 3-4 hours depending upon the number of samples run.
Laboratory Testing
Molecular Testing
Molecular testing is used to detect MPN-specific mutations.
Myeloproliferative neoplasms (MPNs) arise from issues stemming from the bone marrow which lead to abnormally high numbers of certain blood cell types in the blood. Classic MPNs include disorders such as polycythemia vera (PV; Red blood cells), essential thrombocythemia (ET; Platelets), and primary myelofibrosis (PMF; Fibers and blasts).1 Specific variants in the JAK2, CALR, and MPL genes are useful biomarkers for these diseases as they can play a role in either disease diagnosis or provide information regarding disease prognosis.2-6 These essentially mutually exclusive variants occur in a relatively high frequency, as 98% of sample
with PV and 50-65% of samples with ET or PMF exhibit mutations in JAK2. Variants in CALR and MPL are observed in approximately 20-25% or 5-7% of both ET and PMF samples respectively, with only 10-15% of these samples exhibiting triple-negative morphology.1
References:
- Brown, V., S. Borinstein, D. Friedman. 2018. JAK2. My Cancer Genome https://www.mycancergenome.org/content/disease/myeloproliferative-neoplasms/jak2/?tab=0 (Updated April 3)
- Xia, D. and Hasserjian, R. P. (2016), Molecular testing for JAK2, MPL, and CALR in myeloproliferative neoplasms. Am. J. Hematol., 91: 1277-1280. doi:10.1002/ajh.24578
- I. Panovska-Stavridis, A. Eftimov, M. Ivanovski, A. Pivkova-Veljanovska, L. Cevreska, S. Hermouet, et al. Essential thrombocythemia associated with germline JAK2 G571S variant and somatic CALR type 1 mutation
- Clin. Lymphoma Myeloma Leuk., 16 (2016), pp. e55-e57
- Marchioli R., Finazzi G., Specchia G., Cacciola R., Cavazzina R., Cilloni D. Cardiovascular events and intensity of treatment in polycythemia vera. N. Engl. J. Med. 2013;368:22–33. – PubMed
- Plo, Isabelle, and Caroline Marty. “Not just another kinase mutation!” Blood 134.26 (2019): 2335-2337