1 Myeloproliferative Neoplasms, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology
Abstract
The evaluation of patients with persistent or marked thrombocytosis includes molecular testing for the presence of underlying myeloproliferative neoplasms (MPN). This has traditionally included testing initially for JAK2 V617F mutation. Reflex testing of other MPN driver mutations; JAK2 Exon 12, Exon 13, MPL and CALR; are only performed if the JAK2 V617F test result is negative. This case study of a patient with a dual mutation of JAK2 V617F & CALR highlights the diagnostic utility and advantages of testing for MPN driver mutations as a comprehensive panel.
Background
A 72-year-old female presented to her primary care physician with non-specific complaints of dizziness and mild bruising. Routine laboratory testing included a CBC which showed marked thrombocytosis (Platelet Count 911,000). The rest of the CBC was not remarkable. The clinician suspected the possibility of a myeloproliferative neoplasm (MPN), and a blood sample was collected and sent to Precipio’s laboratory for a HemeScreen MPN panel.
Case Work Up &Results:
Results of the HemeScreen® MPN panel were as follows:
• Positive for JAK2 V617F point mutation
• Negative for JAK2 exon 12 mutations
• Negative for JAK2 exon 13 (G571S) mutation
• Negative for MPL W515L/K point mutations
• Positive for CALR exon 9 insertion/deletion mutations
Clinical Implications:
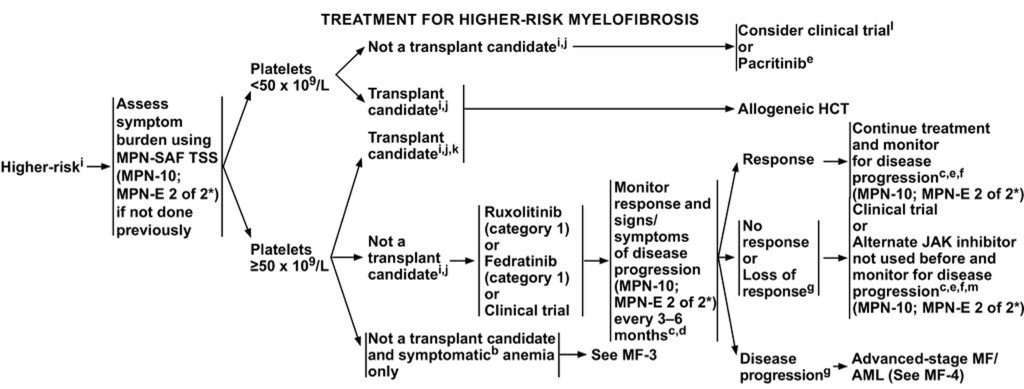
Recent NCCN guidelines suggest that treatment options for this patient will be significantly affected with the additional information of the patient’s dual mutation. The chart1 below details these guidelines.
Diagnosis Provided By Frank Bauer, MD:
Precipio Medical Director. Former Director of Hematopathology at Walter Reed Hospital for the Armed Forces Institute of Pathology. Dr. Bauer taught medical students at UCONN and Quinnipiac Medical Schools.

This presentation is intended for educational purposes only and does not replace independent professional judgment. This document contains proprietary information belonging to Precipio, Inc. No use or disclosure of the information contained herein is permitted without the prior written consent of Precipio Inc.
